G6PD Deficiency
Let us undestand the functions of red blood cell first !
The important function of RBC is binding, transport and deliver of oxygen to all tissues. This is possible when rbc’s are capable of passing through microcapillaries!- which it does through its unique shape and due to loss of nucleus and organelles before entering the circulation from marrow.
RBCs Cannot generate energy by Krebs cycle( oxidative) Due to absence of nucleus , mitochondria and other organnelles and hence they depend on anerobic conversion of glucose and generate energy.
The pathways involved are:
Embden-Meyorhof pathway( EMP or direct glycolytic pathway)– 90% of glucose is catabolized by this pathway
Hexose monophosphate pathway (HMP), or pentose phosphate shunt)- 10% of glucose is catabolized by this pathway
Numerous enzymes are involved in these pathways.
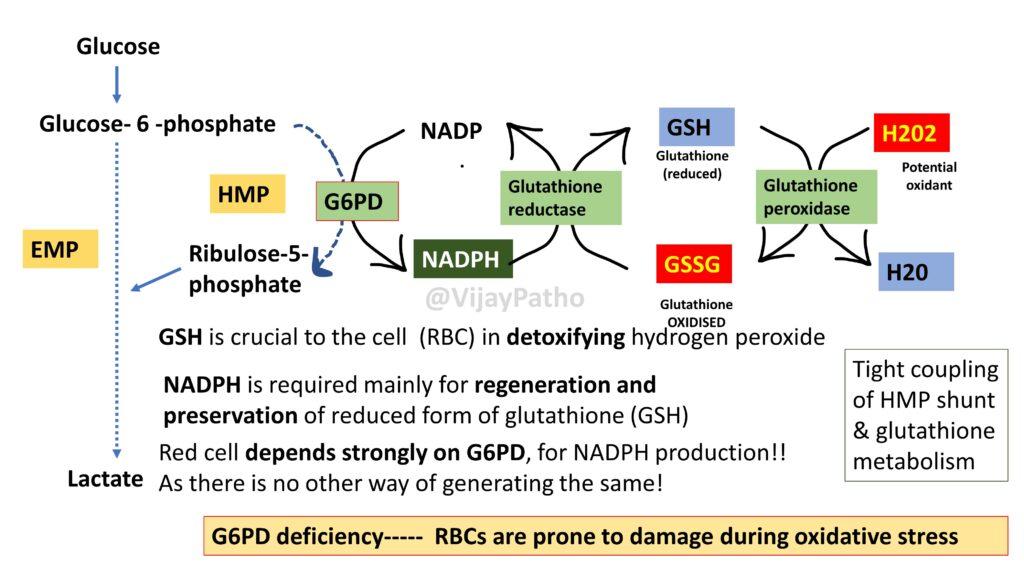
The key enzymes involved in HMP shunt are: Glucose 6 phosphate dehydrogenase (G6PD) &
6-phosphogluconate, dehydrogenase wher 2 moles of NADPH is generated which is the Reducing power!
NADPH is required mainly for regeneration and preservation of reduced form of glutathione (GSH). GSH is crucial to the cell (RBC) in detoxifying hydrogen peroxide which is the free radical and can damage red blood cells.Red cell depends strongly on G6PD, for NADPH production!! As there is no other way of generating the same!
the same is illustrated as below, which explains the Tight coupling of HMP shunt & glutathione metabolism.
Hence in G6PD deficiency—– RBCs are prone to damage during oxidative stress
Glucose-6-Phosphate Dehydrogenase Deficiency
G6PD deficiency is a Hereditary disease, with X linked recessive inheritance resulting in deficient or impaired enzyme function which reduces the ability of red cells to protect themselves against oxidative injuries and lead to hemolysis.
Note that The gene for G6PD is located on long arm of X chromosome. Males are affected and females are carriers.
This is the most common enzymopathy , Estimated to affect over 400 million people worldwide.
Several hundred G6PD genetic variants exist.
The WHO classification as per WHO is illustrated in the table below
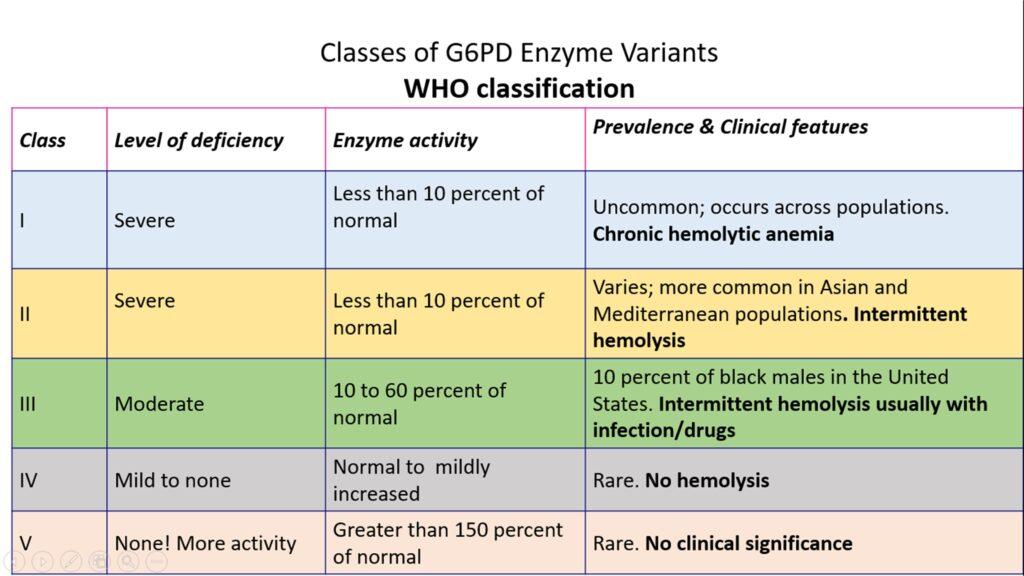
However, only two variants of G6PD are associated with significant clinical hemolytic anemia
1. G6PD A- WHO class III – seen in 10% of American blacks. Here the half-life of G6PD– is moderately reduced ( 13 Days as compared to normal 62 days ) Hence Hemolysis is mild and generally limited to older deficient RBCs
2. G6PD MediterraneanWHO class II : is prevalent in the Middle East.Markedly abnormal. Half life in Hours! and hence Entire RBC population is susceptible and can lead to severe hemolytic anemia
G6PD deficiency and Malaria
High incidence of G6PD deficiency among population in which malaria was once endemic
Plasmodium species do not have any antioxidant mechanisms to tackle oxidative stress!In G6PD deficiency there is accumulation of oxygen derived free radicals inside red blood cell! As described earlier. Hence Deficiency offers resistance to malarial infection
Hemolysis Can be intravascular or extravascular,Episodic, and caused by exposures that generate oxidant stress whcih include
a. Infections – eb- Viral hepatitis, pneumonia, and typhoid fever
b. Drugs – Many drugs, including antimalarials (e.g., primaquine and chloroquine), sulfonamides, nitrofurantoins
c. Foods– Fava bean, which generates oxidants when metabolized. Favism. This is is endemic in the Mediterranean, Middle East, and parts of Africa where consumption is prevalent.
Pathogenesis of findings in peripheral smear as illustrated below.
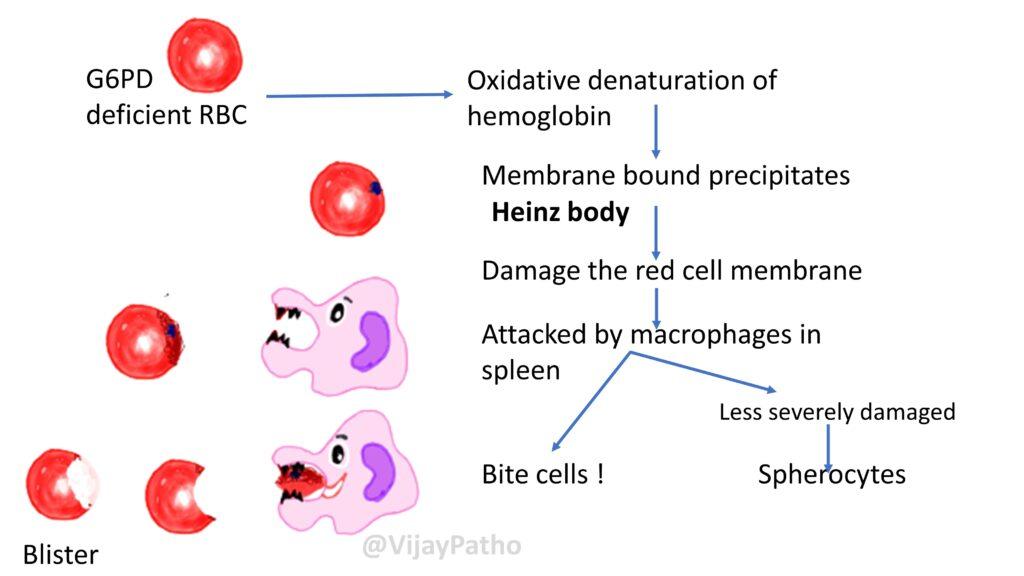
Clinical features
Most patients no symptoms unless exposed to oxidative stress
Manifestations include
1. Acute hemolytic anemia – Sudden onset of jaundice, pallor or dark urine with/without abdominal pain after exposure to stressors(drugs). Ends spontaneously after 1 week, Hb returns to normal
2. Chronic non spherocytic hemolytic anemia – Anemia and jaundice in newborn period. Beyond infancy symptoms are subtle and inconstant. Can have have life long hemolysis in the absence of infection or drug exposure
3. Neonatal jaundice – Rarely present at birth. Peak incidence in 2- 3 days. More jaundice than anemia. If untreated, hyperbilurubinemia may lead to kernicterus with sevee neurologic injury and death
4. Favism – Individuals fall ill after consumption of fava/ broad beans. Most common in children, ages of 1- 5 yrs.Symptoms of acute intravascular hemolysis within 5 to 24 hrs after ingestion of beans. Headache, nausea, chills, fever followed by hemoglobinuria, anemia and jaundice
MORPHOLOGY / LAB FEATURES
Complete blood count – Normocytic normochromic anemia, Reticulocytosis
Urine – Hemoglobinuria
Serum – Increased serum bilirubin
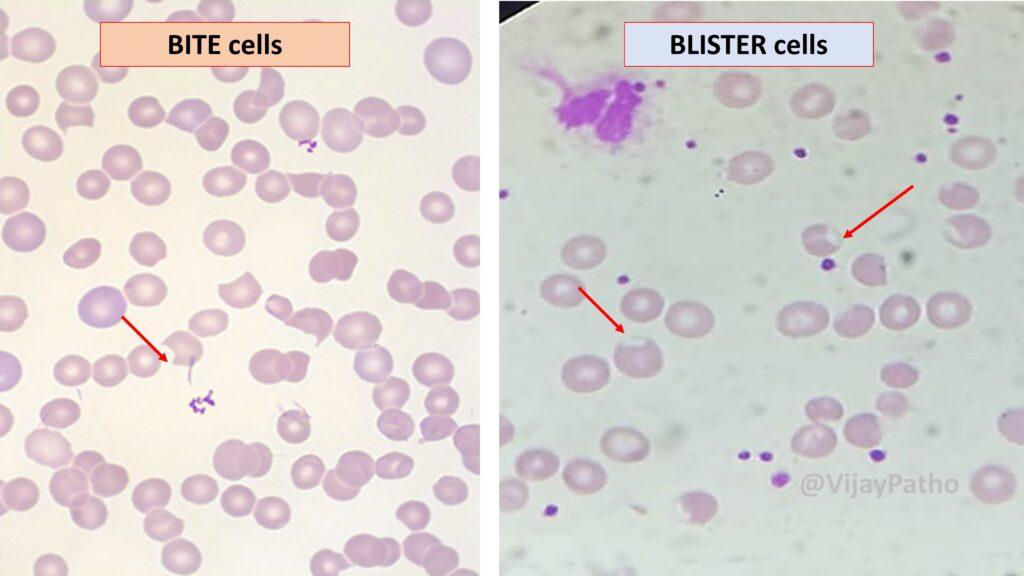
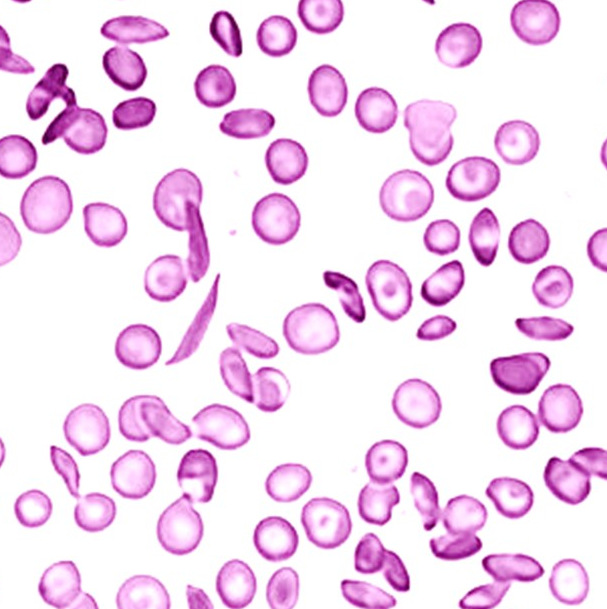
Peripheral smear showing Bite cells and Blister cells
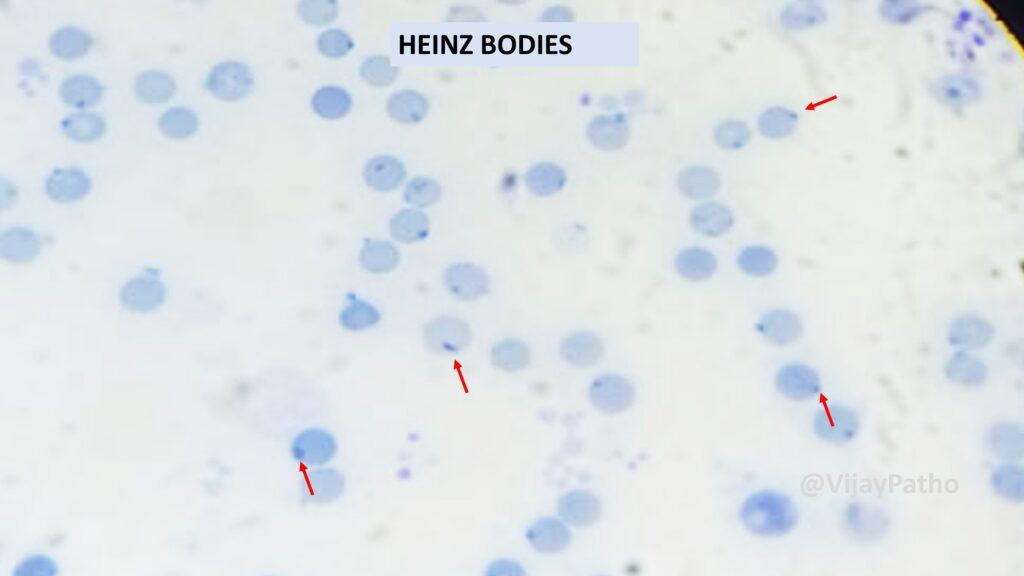
Supravital stain demonstrating Heinz bodies
TESTS
SCREENING tests-
a. Fluorescent spot test – Detects NADPH fluorescence under UV light. Reliable and sensitive
Cells with normal G6PD activity fluoresce due to presence of NADPH
b. Methemoglobin dye reduction test
CONFIRMATORY tests
Spectrophotometric assay of G6PD activity
Note that False negative reactions if most severe enzyme deficient RBCs are removed by hemolysis!
Treatment
Depends on clinical syndrome associated
Supportive care/ removal of triggering agents! treat hyperbilurubinemia, intections etc.
Blood transfusions in severe hemolysis and Hemodialysis if renal damage
Click here to view the video tutorial on G6PD deficieny






Recent Comments