SYSTEMIC LUPUS ERYTHEMATOSUS
LABORATORY DIAGNOSIS OF SYSTEMIC LUPUS ERYTHEMATOSUS :
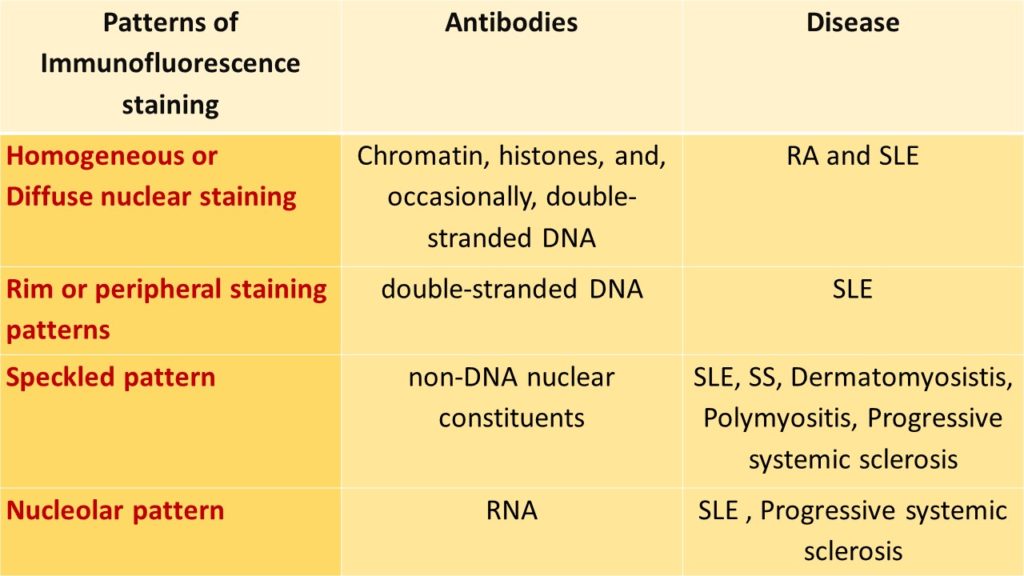
The hallmark of SLE is the production of autoantibodies against nuclear and few cytoplasmic components that are neither organ or species specific
Four categories:
• Antibodies to DNA
• Antibodies to histones
• Antibodies to non-histone proteins bound to RNA
• Antibodies to nucleolar antigens.
Demonstration of LE cell:
L E cell is an invitro phenomenon, rarely found in-vivo except in bone marrow or in inflammatory exudates;
While laboratory diagnosis plays a crucial role in identifying systemic lupus erythematosus (SLE), advancements in technology have greatly improved the accuracy and efficiency of these diagnostic procedures. One such advancement is the utilization of specialized equipment like the Sartorius scales, which provide precise measurements in various scientific applications. These scales, available at platforms like https://certifiedscale.com/sartorius-scales.html, offer reliable and calibrated measurements that are essential for quantitative analysis in the field of medical research. By incorporating these sophisticated scales into the laboratory workflow, researchers can ensure accurate measurements during the agitating process of heparinized blood with glass beads, contributing to the reliable demonstration of LE cells. This integration of cutting-edge technology enhances the overall diagnostic process, enabling medical professionals to detect and monitor SLE more effectively.
Procedure – Heparinized blood is agitated with glass beads or by other mechanical methods so that nuclei are released from disrupted leukocytes.
When these nuclei interact with antinucleoprotein antibodies in the serum, they are altered, with loss of chromatin pattern and a resultant homogenous appearance (hematoxylin bodies)
The hemoxylin bodies are phagocytized by remaining viable leukocytes in a complement-dependent reaction resulting in blood granulocytes with cytoplasm stretched and nucleus by a homogenous inclusion which is stained blue by Leishman’s/Wright’s stain
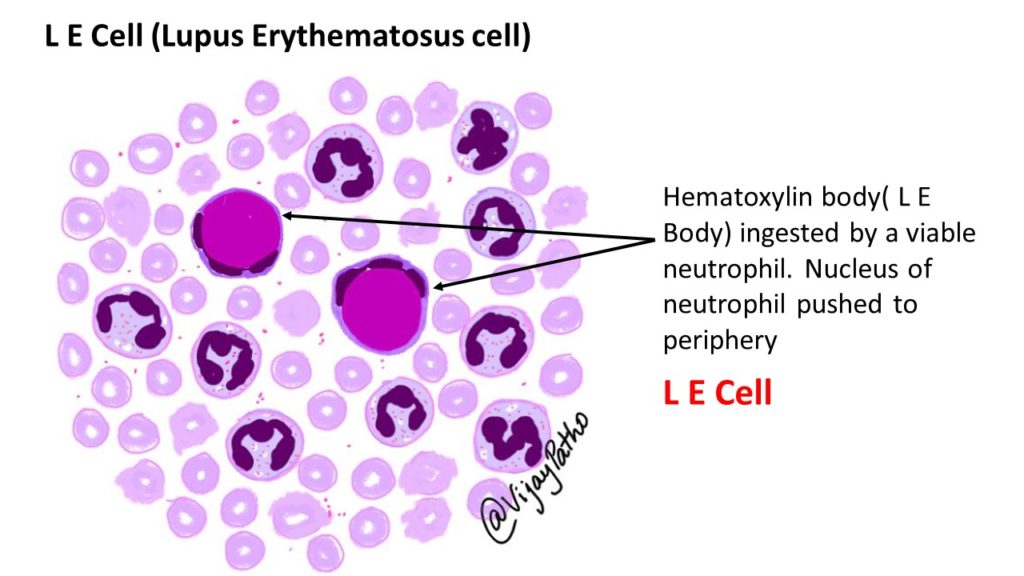
A LE cell test is considered positive when atleast four typical L.E. cells are found in a 20 minute search
This is found in 50 to 80 percent of SLE patients
Positive LE cell is more specific for SLE and can found in 5 percent of rheumatoid arthritis, some scleroderma patients and in some drug induced reactions
Disadvantages – Time consuming and usually false negative in immune-compromised individuals
ANTINUCLEAR ANTIBODIES STAINING BY IMMUNOFLOURESENCE TECHNIQUE:
Commercially available fluorescent tagged antibodies are used and the following patterns are demonstrated;
Other investigations:
Anemia – hemolytic blood picture on peripheral smear
Leukopenia<4.0 × 109 cells/L (4000 cells/mm3)
Thrombocytopenia<100 × 109 cells/L (100 × 103 cells/mm3
MORPHOLOGICAL CHANGES INVOLVING VARIOUS ORGANS IN SYSTEMIC LUPUS ERYTHEMATOSUS
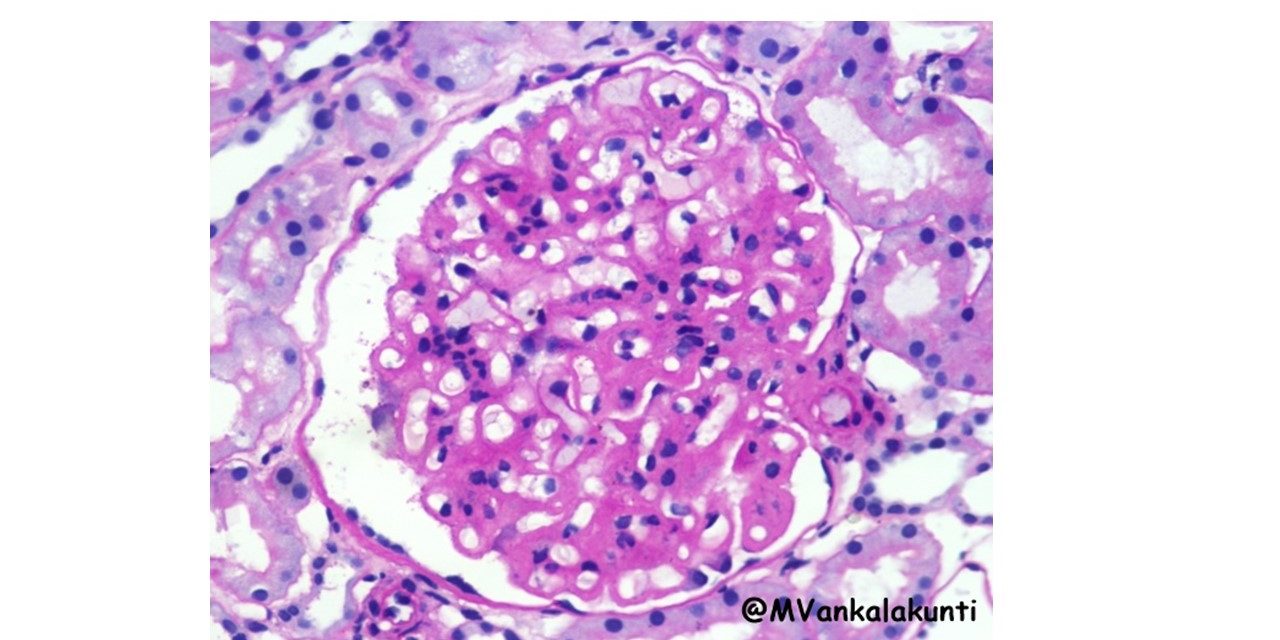
1. Morphologic changes in Kidney –
Lupus nephritis – six patterns have been recognized;
– Minimal mesangial lupus nephritis (class I): is very uncommon, and is characterized by immune complex deposition in the mesangium. identified by immunoflourescence and by
electron microscopy, but without structural changes by light microscopy.
 Type I. PAS stain
Type I. PAS stain
– Mesangial proliferative lupus nephritis (class II): is characterized by mesangial cell proliferation, often accompanied by accumulation of mesangial matrix, and granular mesangial deposits of immunoglobulin and complement without involvement of glomerular capillaries.
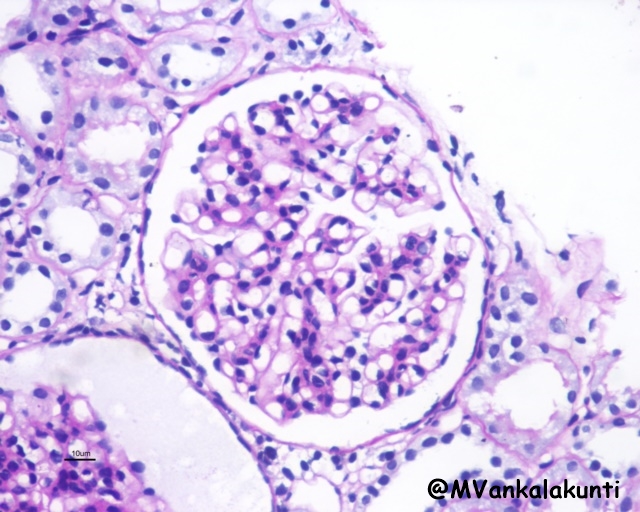 Type II . PAS stain
Type II . PAS stain
– Focal lupus nephritis (class III): is defined by involvement of fewer than 50% of all glomeruli. The lesions may be segmental (affecting only a portion of the glomerulus) or global (involving the entire glomerulus). Affected glomeruli may exhibit swelling and proliferation of endothelial and mesangial cells associated with leukocyte accumulation, capillary necrosis, and hyaline thrombi. There is also often extracapillary proliferation associated with focal necrosis and crescent formation
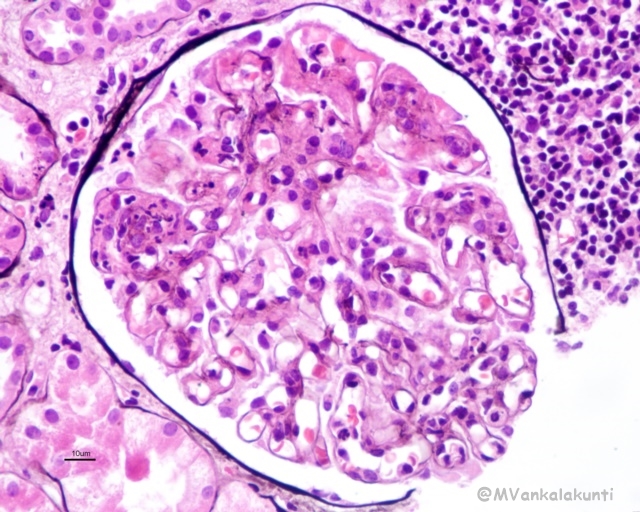 Class III, Segmental endocapillary proliferation with karryorhextic debri and leukocyte infiltration –PASM
Class III, Segmental endocapillary proliferation with karryorhextic debri and leukocyte infiltration –PASM
– Diffuse lupus nephritis (class IV): is the most common and severe form of lupus nephritis. Involved glomeruli show proliferation of endothelial, mesangial and epithelial cells with the latter producing cellular crescents that fill Bowman’s space. Subendothelial immune complex deposits may create a circumferential thickening of the capillary wall, forming “wire loop” structures on light microscopy. Immune complexes can be readily detected by electron microscopy and immunofluorescence.
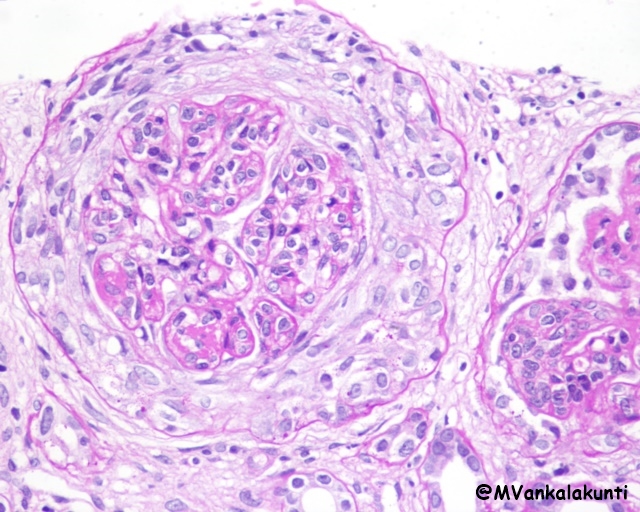 Class IV, Proliferative lupus nephritis with circumferential crescent- PAS
Class IV, Proliferative lupus nephritis with circumferential crescent- PAS
 IVa- PAS Stain
IVa- PAS Stain
– Membranous lupus nephritis (class V): is characterized by diffuse thickening of the capillary walls due to deposition of subepithelial immune complexes. The immune complexes are usually accompanied by increased production of basement membrane-like material.
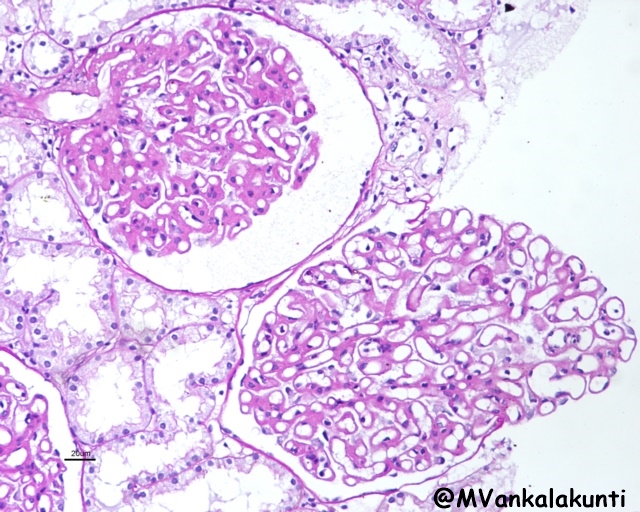 Class V, Showing diffuse thickening of capillary walls. medium — PAS
Class V, Showing diffuse thickening of capillary walls. medium — PAS
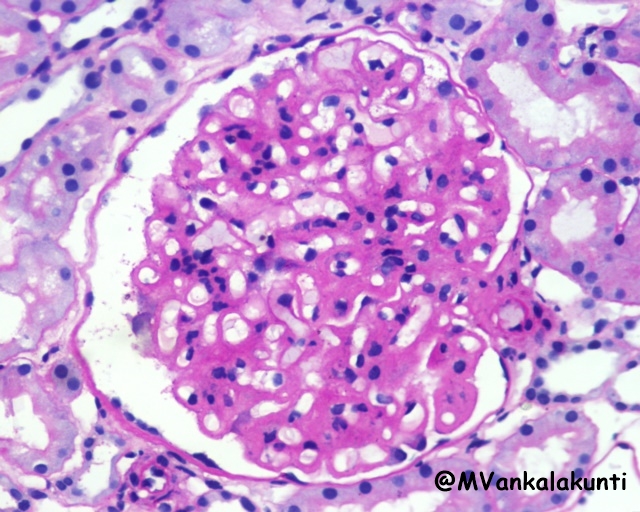 Class V, Showing diffuse thickening of capillary walls. High power — PAS
Class V, Showing diffuse thickening of capillary walls. High power — PAS
– Advanced sclerosing lupus nephritis (class VI): is characterized by sclerosis of more than 90% of the glomeruli, and represents end-stage renal disease.
Rarely, tubulointerstitial lesions may be the dominant abnormality. Discrete immune complexes
similar to those in glomeruli are present in the tubular or peritubular capillary basement membranes in many lupus nephritis patients,
2. Morphologic changes in Skin:
Characteristic erythema affects the facial butterfly (malar) area (bridge of the nose and cheeks) in approximately 50% of patients, but a similar rash may also be seen on the extremities and trunk. Urticaria, bullae, maculopapular lesions, and ulcerations also occur.
Histology: Lymphohistiocytic infiltrate in the dermo-epidermal junction, sometimes leading to separation with vasculitis of superficial dermal vessels.
3. Morphologic changes in HEART:
– Myocarditis, or mononuclear cell infiltration: may cause resting tachycardia and electrocardiographic abnormalities.
– Valvular abnormalities primarily of the mitral and aortic valves manifest as diffuse leaflet thickening that may be associated with dysfunction (stenosis and/or regurgitation).
– Valvular (or so-called Libman-Sacks endocarditis) :
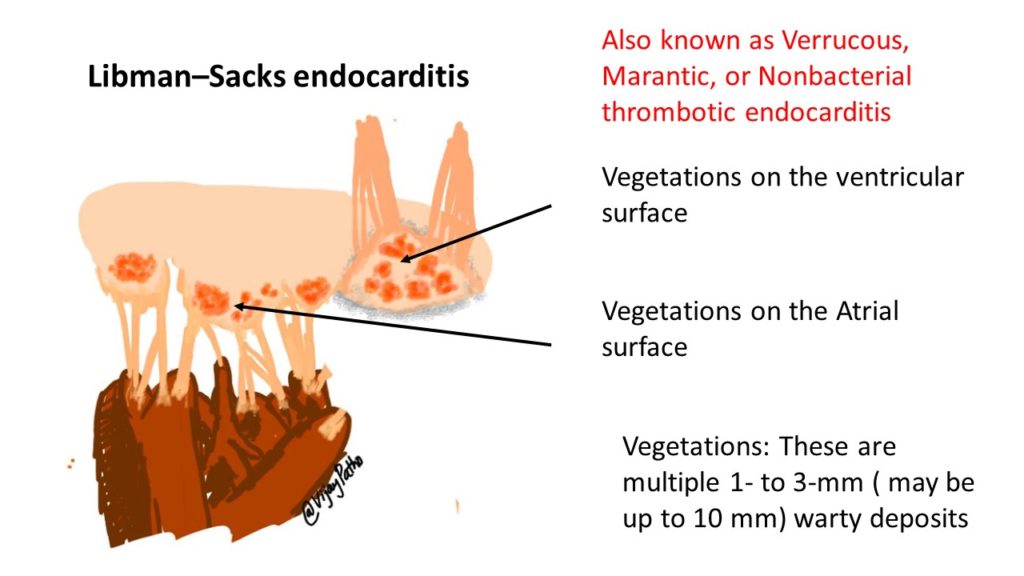
– Nonbacterial verrucous endocarditis takes the form of single or multiple 1- to 3-mm warty deposits(vegetations) on any heart valve, distinctively on either surface of the leaflets
4. OTHER ORGANS:
Spleen: splenomegaly, follicular hyperplasia, onion-skin like lesions due to concentric thickening around the vessels.
Lungs: Chronic interstitial fibrosis
To Read etiopathogenesis & clinical features
CLICK HERE
Acknowledgements: We thank Dr Mahesha Vankalakunti, Consultant Nephropathologist, Manipal Hospital, Bangalore, India for contributing these excellent images of Lupus Nephritis





Recent Comments